28+ Calculating The Ph Of A Buffer
Determine the pH of a buffer solution that contains 0100 M hydrazoic acid pKa 460 p K a 460 and 0100 M lithium azide. From the calculation above the pH of buffer solution is 738.

Buffer Solutions A Guide For A Level Students Ppt Download
For example if a system contains both CH3COOH and CH3COONa then the pH.

. Web This calculator is valid for a buffer of a weak acid and its conjugate base of the same system. These amounts should be. Web The pH scale is used to measure the acidity or basicity of a solution.
Web To calculate the pH of the buffer solution you need to know the amount of acid and the amount of the conjugate base combined to make the solution. List the values you are given. Web Steps for Calculating the pH of a Buffer Step 1.
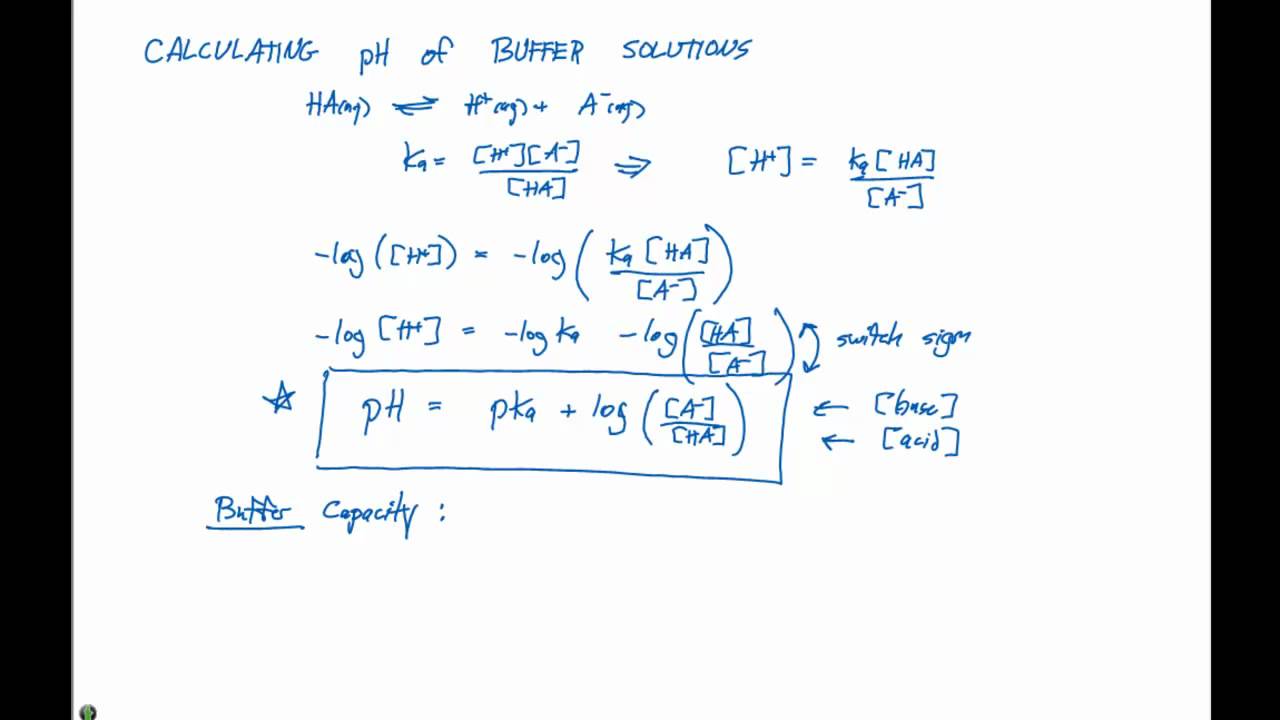
Web The pH is a measure of the acidity or basicity of an aqueous solution. Web Calculation of the pH of a buffer solution Calculate the pH of a buffer solution formed by adding 2000 cm 3 of 010 moldm -3 NaOH to 4000 cm 3 of the weak acid HX which. PH pKa log A-HA The Henderson-Hasselbalch equation enables determination of a buffer solutions pH when the pKa is known.
Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt. Find the pH of a buffer solution that. Now lets check our answer to see whether its reasonable.
Web Postby Yixin Wu 3B Sat Jan 28 2023 552 am. PH -log. Web The pH of a buffer is determined by two factors.
Web Calculating pH of buffer. Use the values given in the Henderson-Hasselbalch equation to solve for pH. It ranges from 0 to 14 with 7 being neutral and values above 7 indicating alkaline solutions and.
Web I cant calculate the pH using Henderson-Hasselbalch approximation because concentration of acid is 0. I got a response that I should solve it like this after I. Web Buffer solution pH calculations Chemistry Khan Academy Khan Academy Organic Chemistry 210K subscribers 942K views 8 years ago Example of calculating the pH of.
The pH is defined through the concentration of text H H ions in the solution. Web An acidic buffer solution is simply one which has a pH less than 7. 1 The equilibrium constant Ka of the weak acid and 2 the ratio of weak base A - to weak acid HA in solution.
I am wondering how to calculate the pH of a salt with both weak conjugate acid or base.

Molecules Free Full Text Mass Spectrometric Analysis Of Antibody Epitope Peptide Complex Dissociation Theoretical Concept And Practical Procedure Of Binding Strength Characterization

Calculating The Ph Of A Buffer Youtube

High Resolution Native Mass Spectrometry Chemical Reviews
How To Calculate Ph Of Buffer Solutions Sciencing

Solutions For The Calculation Of Ph In Buffer Systems

Comparison Of The Measured And Calculated Ph Values For Ammonium Buffers Download Scientific Diagram

1 Buffer 2 Ph Ph Log H Or Ph Log H 3 O Example I What Is The Ph Of Solution With H 32 X M L Ph Log H Ph Ppt Download

How To Calculate Ph Of Buffer Solutions Sciencing

Determining The Ph Of A Buffer Solution Example Calculation Youtube

How To Calculate Ph Of Buffer Solutions Sciencing

Molecules Free Full Text Mass Spectrometric Analysis Of Antibody Epitope Peptide Complex Dissociation Theoretical Concept And Practical Procedure Of Binding Strength Characterization
17 2 Calculating Ph Of Buffer Solutions Youtube

2 Buffer Solutions Change In Ph Of A Buffer Youtube

17 2 Calculating Ph Of Buffer Solutions Youtube

How To Calculate The Ph Of A Buffer Solution Fully Worked Example Youtube
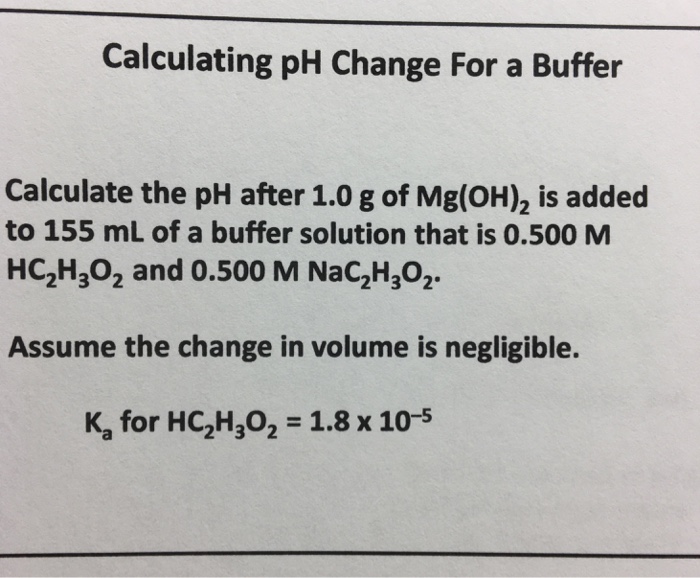
Solved Calculating Ph Change For A Buffer Calculate The Ph Chegg Com

Lo69jroipyq3cm